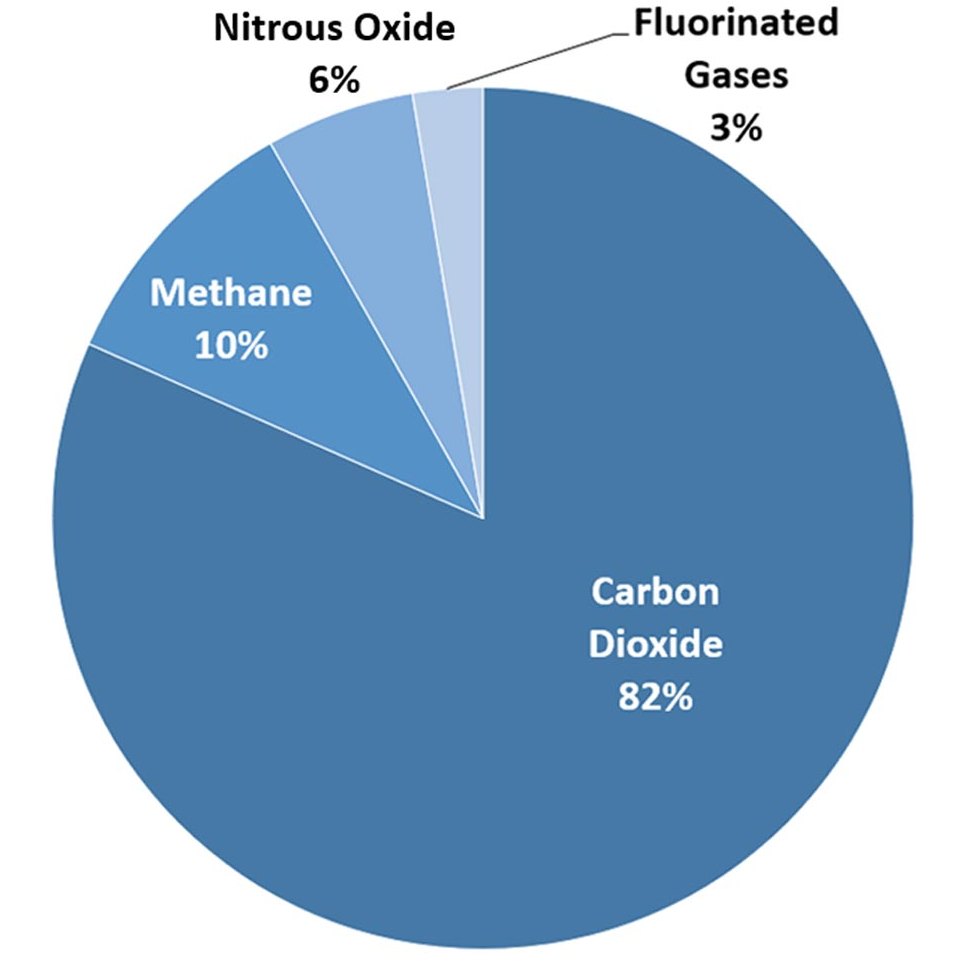
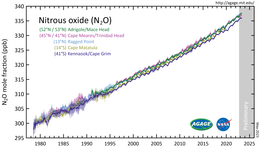
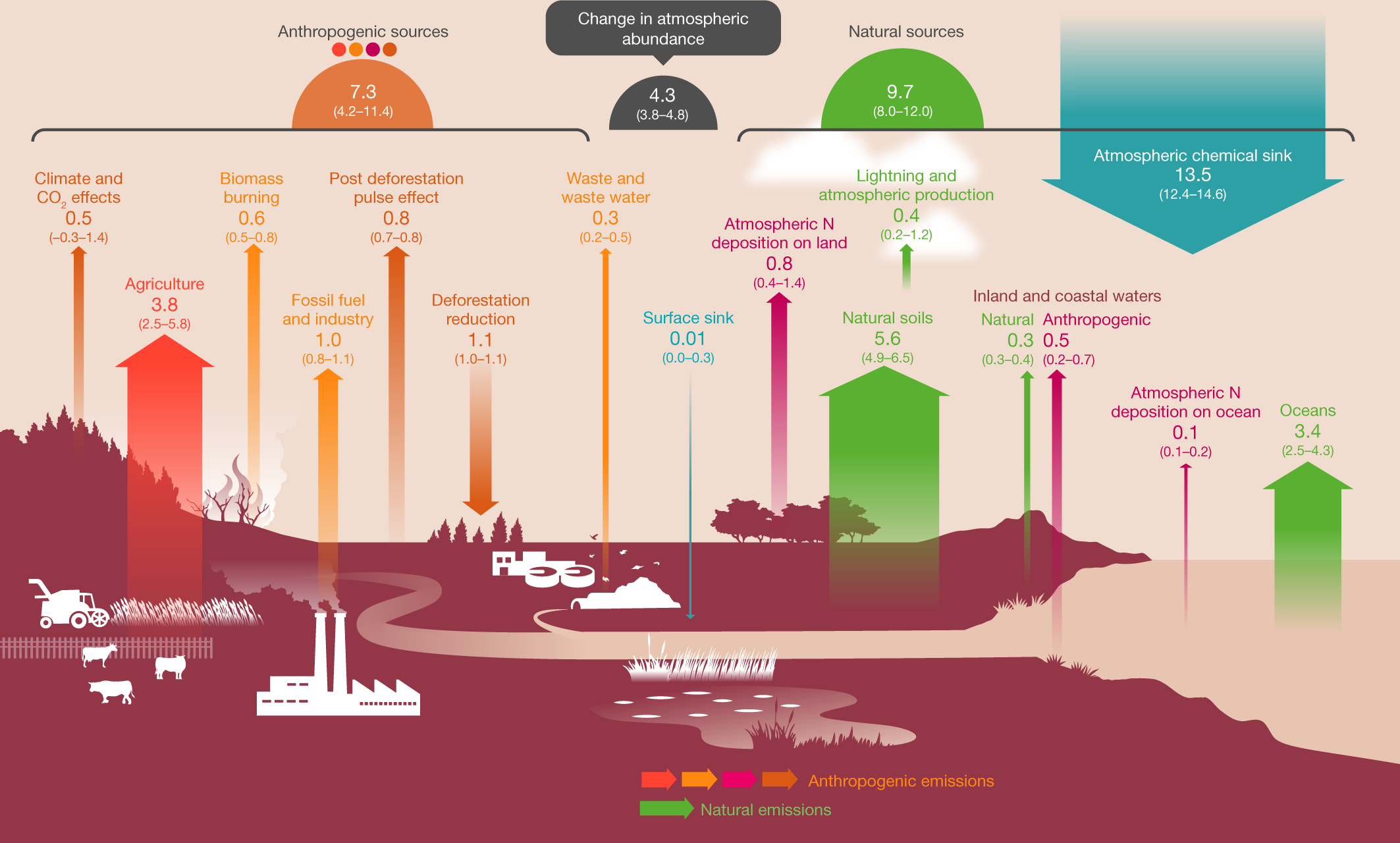
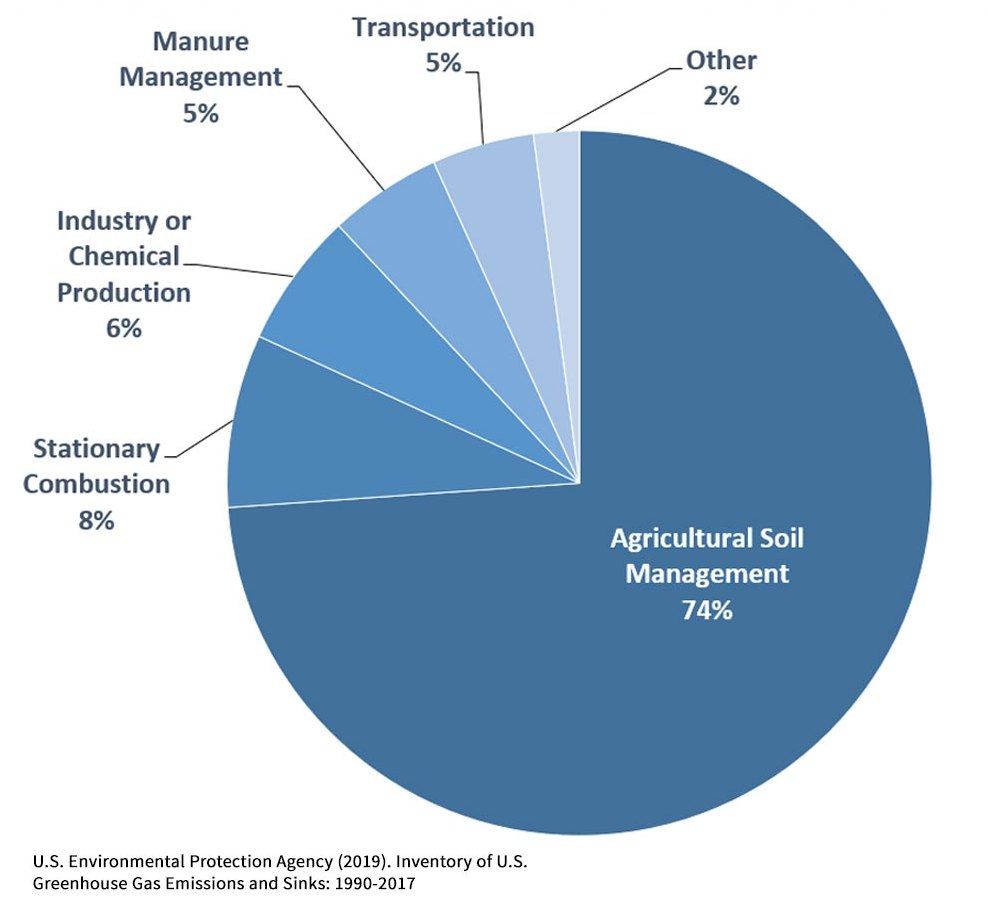
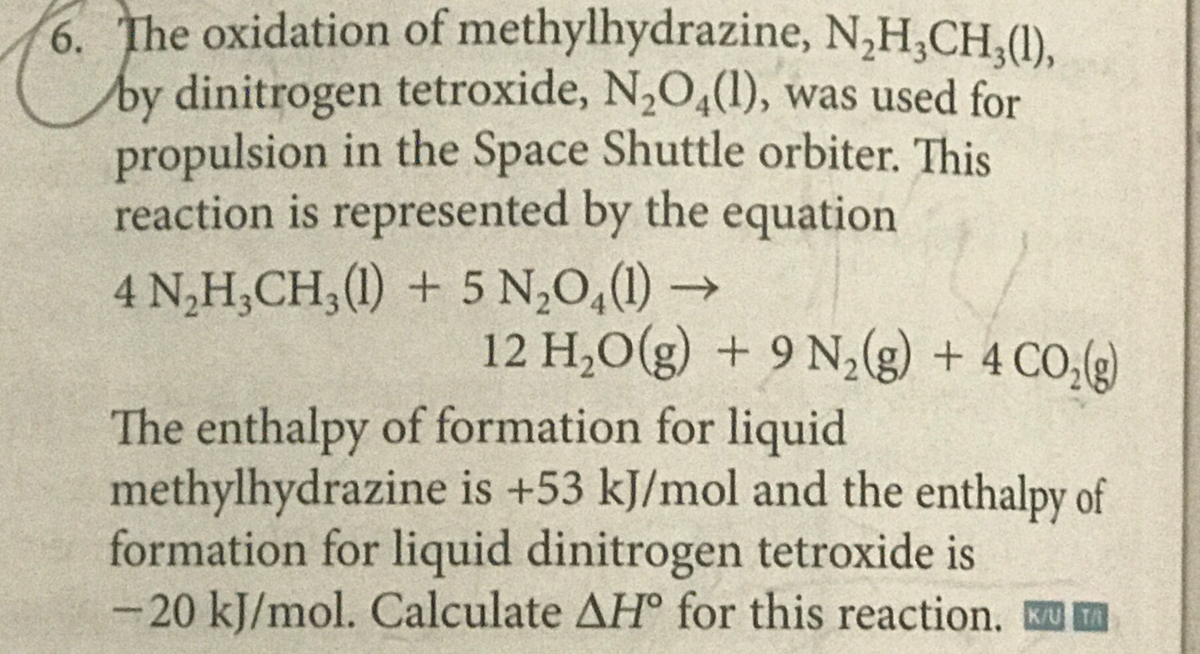Difference Between Nitric Oxide and Nitrous Oxide | Definition, Properties, Reactions, Similarities and Differences

Nitrous Oxide(Laughing Gas): Preparation, Properties, uses and structure | by Chemistry Page | Medium

SOLVED: When ammonia reacts with dinitrogen oxide gas (ΔHf° = 82.05 kJ/mol), liquid water and nitrogen gas are formed. How much heat is liberated or absorbed by the reaction that produces 355
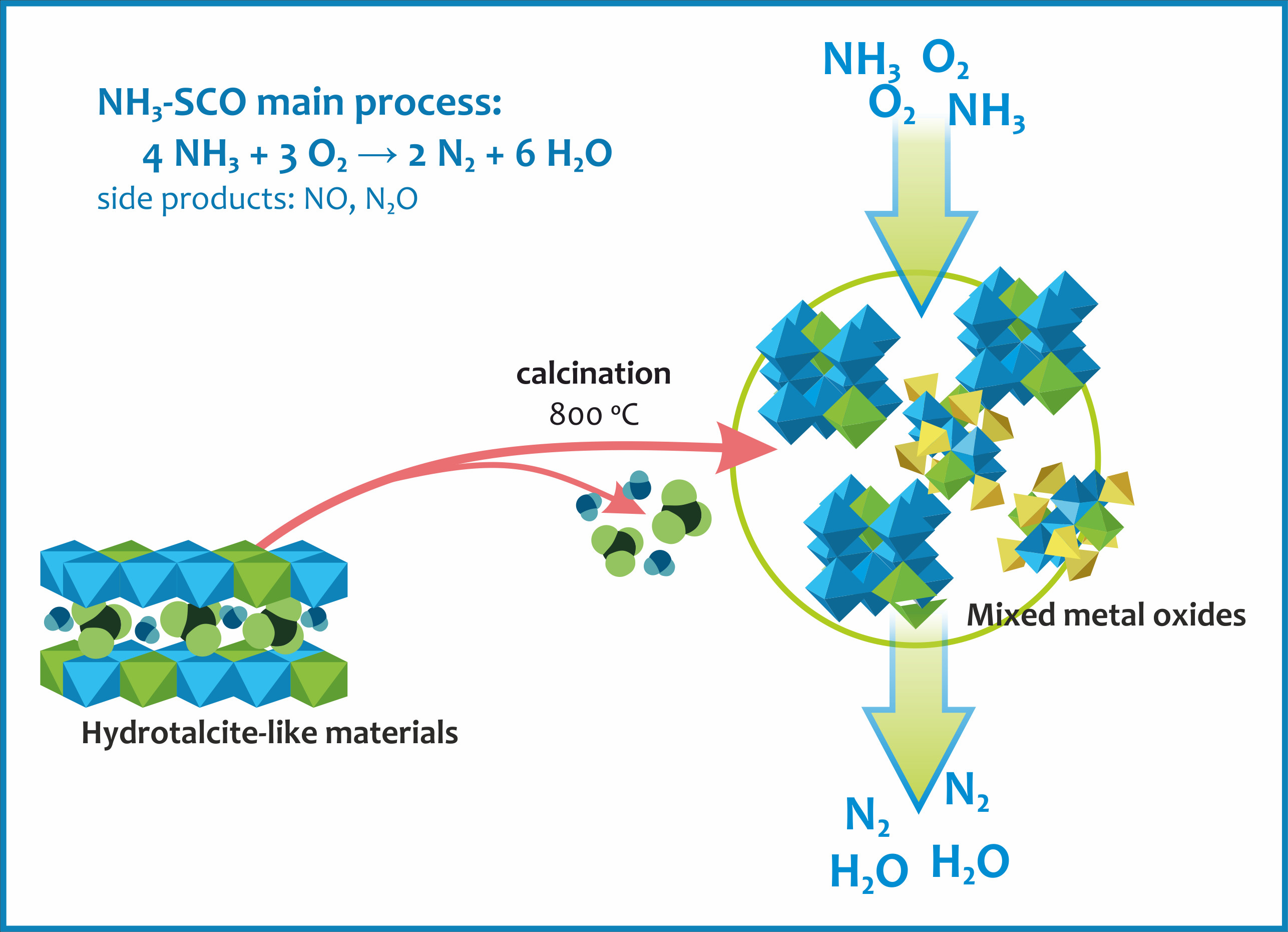
Catalysts | Free Full-Text | Cu-Mg-Fe-O-(Ce) Complex Oxides as Catalysts of Selective Catalytic Oxidation of Ammonia to Dinitrogen (NH3-SCO)

Accelerating ammonia synthesis in a membraneless flow electrolyzer through coupling ambient dinitrogen oxidation and water splitting - ScienceDirect

IJMS | Free Full-Text | Regulation of the Emissions of the Greenhouse Gas Nitrous Oxide by the Soybean Endosymbiont Bradyrhizobium diazoefficiens
![Data for the decomposition of dinitrogen oxide on a gold surface at 900 degrees Celsius are given below. Verify that the reaction is first order by preparing a graph of ln ~[N2O] Data for the decomposition of dinitrogen oxide on a gold surface at 900 degrees Celsius are given below. Verify that the reaction is first order by preparing a graph of ln ~[N2O]](https://homework.study.com/cimages/multimages/16/at16316449417038598771.jpg)
Data for the decomposition of dinitrogen oxide on a gold surface at 900 degrees Celsius are given below. Verify that the reaction is first order by preparing a graph of ln ~[N2O]